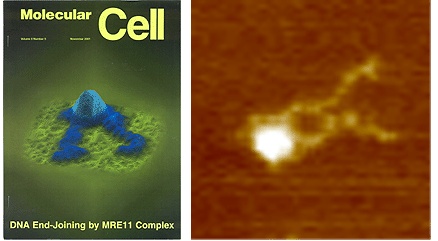
Rad50/Mre11/NBS1 Complex
The human Rad50/Mre11/NBS1 (RMN) play a central role in the first steps of DSB repair and has a very distinct architecture with a central globular domain and two protruding “arms” of 40-50 nm long (Figure 2). Our lab previously revealed by high resolution AFM how DNA binding induces structural changes in the arms of the complex. Currently we are further investigation the role of ATP-hydrolysis in the regulation of DNA binding and tethering.